Carbon and its Compounds notes class 10 | Carbon and its Compounds notes class 10th
Carbon and its Compounds Class 10 notes are available for free download in PDF format. NCERT Class 10 Science Notes Chapter 4 on Carbon and Its Compounds and NCERT Solutions for Class 10 Chapter 4 are also provided here.
Carbon and its Compounds Notes CBSE Class 10 Science Chapter 4
carbon and its compounds class 10 All of the items you mentioned, including food, clothing, medications, books, and many others, are based on the adaptable element Carbon.
Additionally, carbon serves as the basis for all living things. The crust of the earth and the atmosphere both contain very little carbon.
The amount of carbon in the earth’s crust as minerals (such as carbonates, hydrogen carbonates, coal, and petroleum) is just 0.02%, while the amount of carbon dioxide is 0.03%.in the atmosphere.
Despite the limited amount of carbon present in nature, carbon appears to be quite important.
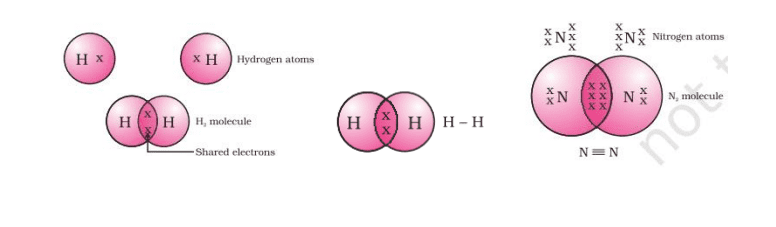
Covalent Bond
We know a carbon bond between two atoms sharing a pair of electrons as a covalent bond. Carbon exists in two free states, the first state is a free state and is a combined state.
Free States Carbon is graphite, diamond and fullerene. Combined States Carbon is Carbon-dioxide, Glucose, Sugar etc.
Diamond
Diamonds form a robust three-dimensional network held together by powerful carbon-carbon covalent bonds. This crystal structure results in diamonds being hard and having high melting points. Although they possess a lustrous appearance in the presence of light, diamonds are poor conductors of electricity. Diamonds find their most popular application in the creation of jewellery. Additionally, they are also employed in the manufacture of cutting and drilling tools.
Graphite
Graphite is formed by weak Van der Waal forces that result from the bonding of each carbon atom with three other carbon atoms to create hexagonal rings. Due to its unique bonding structure, graphite is a highly efficient conductor of heat and electricity. This makes it an ideal material for use as a dry lubricant in machine parts and as a component of lead pencils.
Fullerene
This structure is spherical in shape and has a hollow cage-like appearance. Its composition is comparable to that of fullerene, however, it is characterized by the presence of not only hexagonal but also pentagonal or heptagonal rings.
Types of Covalent Bonds
Single Covalent Bond: when two atoms in a molecule share a single pair of electrons.
For example; F2, Cl2, H2 etc.
Double Covalent Bond: When two atoms share two pairs of electrons in a molecule.
For example; O2, CO2 etc.
Triple Covalent Bond: When three pairs of electrons are shared
between two atoms in a molecule. For example; N2 etc.
Versatile Nature of Carbon Atoms:
The carbon atom’s two key characteristics allow for the formation of a huge variety of compounds.
CATENATION:
Catenation is the ability of a carbon atom to make bonds with other carbon atoms. Similar to carbon, silicon may contain up to seven or eight hydrogen atoms in combination.
TETRAVALENCY:
As we are all aware, the atom of carbon has four valence electrons.
Carbon creates a covalent bond by sharing its four valence electrons with other atoms because it cannot gain or lose one of its valence electrons to achieve an octet.
Saturated and Unsaturated Carbon Compounds

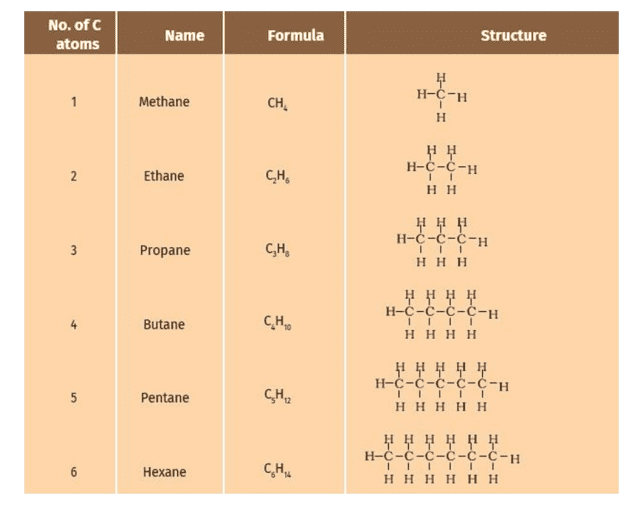
Formulae and Structures of Saturated Compounds of Carbon and Hydrogen
Saturated hydrocarbons, also known as alkanes, are compounds composed solely of carbon and hydrogen with single bonds between the carbon atoms.
The general formula for alkanes is CnH2n+2, where “n” represents the number of carbon atoms in the molecule.
The structure of saturated hydrocarbons can be represented using line or structural formulas. In a line formula, each vertex or line ending represents a carbon atom, and the attached hydrogen atoms are implied. In a structural formula, all bonds and atoms are explicitly shown.
| Hydrocarbon | Formula | Structural Formula |
|---|---|---|
| Methane | CH₄ | H-C-H |
| Ethane | C₂H₆ | H₃C-CH₃ |
| Propane | C₃H₈ | H₃C-CH₂-CH₃ |
| Butane | C₄H₁₀ | H₃C-CH₂-CH₂-CH₃ |
| Pentane | C₅H₁₂ | H₃C-CH₂-CH₂-CH₂-CH₃ |
| Hexane | C₆H₁₄ | H₃C-CH₂-CH₂-CH₂-CH₂-CH₃ |
| Heptane | C₇H₁₆ | H₃C-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃ |
| Octane | C₈H₁₈ | H₃C-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃ |
| Nonane | C₉H₂₀ | H₃C-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃ |
| Decane | C₁₀H₂₂ | H₃C-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₂-CH₃ |
In these formulas, H₃C represents the methyl group (-CH₃), and the carbon atoms are arranged in a straight chain. The structural formulas indicate the single bonds between carbon atoms and the implied hydrogen atoms to satisfy the valency of each carbon atom.
Chemical Properties of Carbon Compounds
Combustion:
- Carbon and its compounds are utilised as fuels because they produce a lot of heat energy when they burn in the air.
- Saturated hydrocarbons typically burn in the air with a blue, clean flame.
- Because unsaturated hydrocarbon has a higher percentage of carbon than saturated hydrocarbon, which does not completely oxidise in air, it burns in the air with a yellow sooty flame.
- Sulphur and nitrogen oxides generated by the burning of coal and petroleum are what causes acid rain.
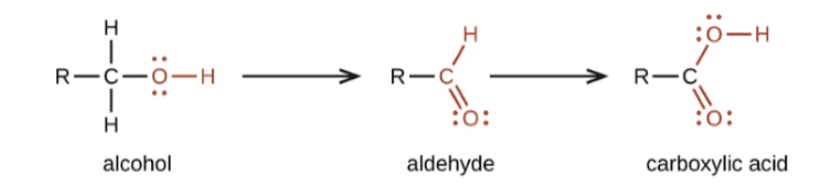
Oxidation
- The Carbon compounds easily oxidised. In addition to this full oxidation, there exist processes that turn alcohols into carboxylic acids.
- Alcohols can become carboxylic acids when an oxidising agent like alkaline potassium permanganate (KMnO4) or acidic potassium dichromate is present.
Addition Reaction
- In the presence of catalysts like palladium or nickel, unsaturated hydrocarbons add hydrogen to produce saturated hydrocarbons.
- Catalysts are substances that speed up or slow down reactions without changing the course of the reaction itself. Many people frequently use this reaction to hydrogenate vegetable oils.
- Animal fats typically contain saturated fatty acids, which experts consider harmful to health. Oils containing unnormal fatty acids should be a choice for cooking.

Substitution Reaction
- hydrocarbons are fairly unreactive and inactive in the presence of the majority of reagents.
- However, in the presence of sunlight, chlorine rapidly reacts and combines with hydrocarbons, actively substituting one hydrogen atom at a time through a replacement reaction and replacing either a single atom or a group of atoms with another atom.
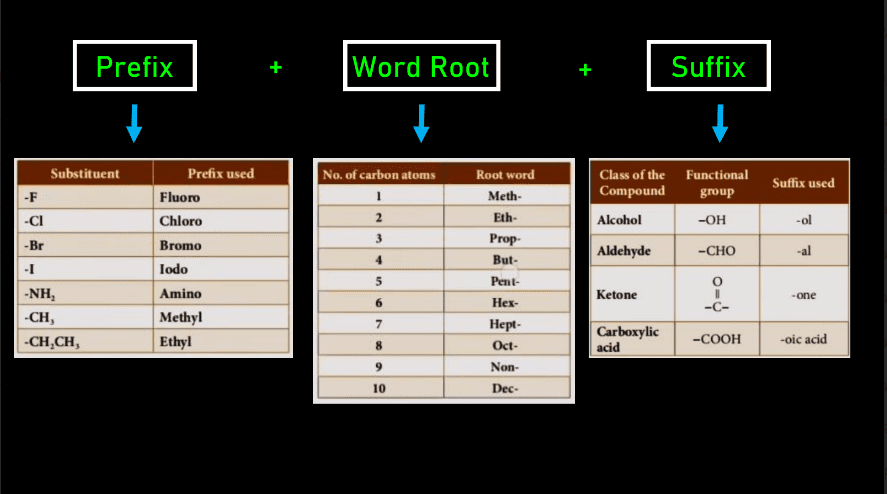
(Naming of carbon compounds)
- Step1. Identify the longest chain of carbon.
- Step2. Give the base name of the compound depending on the quantity of carbon.
- Step3. Adding Either a Prefix or suffix to the base.
- Step4. Name the substituent present.
CARBON AND ITS COMPOUNDS IN BRIEF
Carbon is a versatile non-metal.
- Hydrocarbons can have a cyclic branch or straight chain structure structurally.
- Carbon can form single, double and triple covalent bonds.
- The carbon atom shares electrons with the atoms of other non-metals like oxygen, nitrogen, hydrogen, and chlorine.
- Isomerism results from a difference in the structural arrangement of the identical molecule.
- A homologous series is a group of substances that share a general formula and have similar chemical characteristics but diverse physical characteristics.
- Carbon-based compounds are excellent fuels.
- Ethanol is an important industrial compound. It reacts with reactive metals and also dehydrates to ethene.
- Ethanoic acid is another important compound. It combines with ethanol to form sweet-smelling esters
SOAPS AND DETERGENTS
- Soap molecules consist of long-chain carboxylic acid salts in the form of sodium or potassium.
- Soap interacts with oil through its carbon chain, while it interacts with water through its ionic end.
- The soap molecules organize into micelle-like structures (see Figure) with one end directed into the oil droplet and the other end directed towards the outside, resulting in the creation of an emulsion in water.
- Thus, the soap micelle helps remove dirt from water, enabling us to wash our clothes thoroughly.
- The reaction between the soap molecule and the magnesium and calcium salts found in hard water produces an insoluble substance called scum. This muck makes cleaning difficult.

- Soaps and detergents are both cleaning agents that are used for removing dirt and stains from surfaces and fabrics.
- Soaps are made by the saponification of fats and oils with an alkali such as sodium hydroxide or potassium hydroxide.
- Detergents are synthetic substances that are made from petrochemicals and have similar cleaning properties to soaps.
- Detergents are less likely to leave residues on surfaces and clothing, making them more suitable for washing machines and dishwashers.
- Soaps are less effective in hard water, as they form insoluble scum with the calcium and magnesium ions present in hard water.
- Soaps have a higher pH than detergents, making them harsher on the skin and more likely to cause irritation.
- Detergents have a neutral pH, making them gentler on the skin.
- Soaps and detergents have a hydrophilic (water-loving) head and a hydrophobic (water-repelling) tail, which allows them to surround and emulsify dirt and grease, making them easier to remove.
- Soaps and detergents work by reducing the surface tension of water, allowing it to penetrate fabrics and surfaces more effectively.

Class 10 Science Notes
Chapter of class 10 science basically includes some important Chapter topics from the NCERT Book, Such as acids bases and salts, metals and non-metals, chemical reactions and equations, Carbon and its Compounds, Life Processes, Control, and Coordination, etc.
Chapter-wise notes on these subjects are available in the table below, click on the links to access them.
- Chapter 1 Chemical Reactions and Equations Class 10 Notes
- Chapter 2 Acids Bases and Salts Class 10 Notes
- Chapter 3 Metals and Non-metals Class 10 Notes
- Chapter 4 Carbon and its Compounds Class 10 Notes
- Chapter 5 Periodic Classification of Elements Class 10 Notes
- Chapter 6 Control and Coordination Class 10 Notes
- Chapter 7 How do Organisms Reproduce Class 10 Notes
- Chapter 8 Heredity and Evolution Class 10 Notes
- Chapter 9 Light Reflection and Refraction Class 10 Notes
- Chapter 10 Human Eye and Colourful World Class 10 Notes
- Chapter 11 Electricity Class 10 Notes
- Chapter 12 Magnetic Effects of Electric Current 10 Notes
- Chapter 13 Our Environment Class 10 Notes
CBSE Class 10 Chapter 4 Carbon and Its Compounds FAQs
A: Carbon is essential for the formation of organic compounds as it can form covalent bonds with other carbon atoms and other elements such as hydrogen, oxygen, nitrogen, and sulfur.
This property allows for the creation of a vast variety of complex organic molecules that make up living organisms and many useful materials.
A: Homologous series refers to a group of organic compounds that have similar chemical properties and differ from each other by a common structural unit.
Members of a homologous series have the same functional group and exhibit similar physical properties such as melting and boiling points.
A: Carbon compounds are covalently bonded, meaning they share electrons between atoms. This results in compounds that generally have lower melting and boiling points, are non-conductive and are generally more flammable than ionic compounds.
Ionic compounds, on the other hand, have a higher melting and boiling point, are conductive when in a liquid or aqueous state, and are less flammable than most carbon compounds.
A: The molecular formula of a compound is determined by knowing the number and type of atoms present in the molecule. This can be determined through chemical analysis, such as mass spectrometry or infrared spectroscopy.
From this information, the molecular formula can be deduced using the periodic table and knowledge of chemical bonding.
A: Isomers are compounds that have the same molecular formula but different structural formulas. They may have different physical and chemical properties due to differences in the arrangement of atoms in the molecule. For example, butane and isobutane are isomers, both having the molecular formula C4H10, but their structures are different.

