The Class 10 periodic table is a fundamental concept in chemistry that allows us to organize and understand the properties of different elements.
This article will explore the historical development of the periodic table, its modern structure, and the key trends observed within it.
The periodic classification of elements is the systematic arrangement of elements based on their atomic number, electron configuration, and recurring chemical properties.
It provides a framework for understanding the relationships between various elements and predicting their behaviour. Unlock in-depth Class 10 periodic table Classification of Elements
John Newlands’ Octaves
In the mid-19th century, John Newlands proposed the concept of “octaves” to classify elements.
He arranged the known elements in increasing order of atomic weights and observed that every eighth element showed similarities in properties.
However, this approach had limitations as it failed to accommodate all elements and did not provide a clear pattern.
Dmitri Mendeleev’s Periodic Table
The present periodic table is due to the Russian chemist Mendeleev.
The periodic function of an element’s atomic mass determines its properties. The periodic table created by Mendeleev is based on the chemical makeup of elements.
contain seven horizontal rows called periods and eight vertical columns called groups that make up Mendeleev’s periodic table.
Achievements of Mendeleev’s Periodic Table
- Grouping together elements with similar properties could be done.
- We left some gaps for the undiscovered elements.
- Placing noble gases without disturbing the existing order could be accomplished.
Limitations:
- No fixed position for hydrogen
- No place for isotopes
- No regular trend in atomic mass
Modern Periodic Table
A periodic function of an element’s atomic number determines its properties.
The number of protons in the atomic nucleus is indicated by the symbol Z and is known as the atomic number.
- Seven horizontal rows and 18 vertical columns, collectively known as groups and periods, make up the modern periodic table.
- Valence electrons are found in a group of elements.
- As we move along the group, there are more shells available.
- A period’s elements all have the same number of shells.
- A fresh electronic shell is filled with each period.
- How electrons are put into different shells determines how many elements are inserted in a given time.
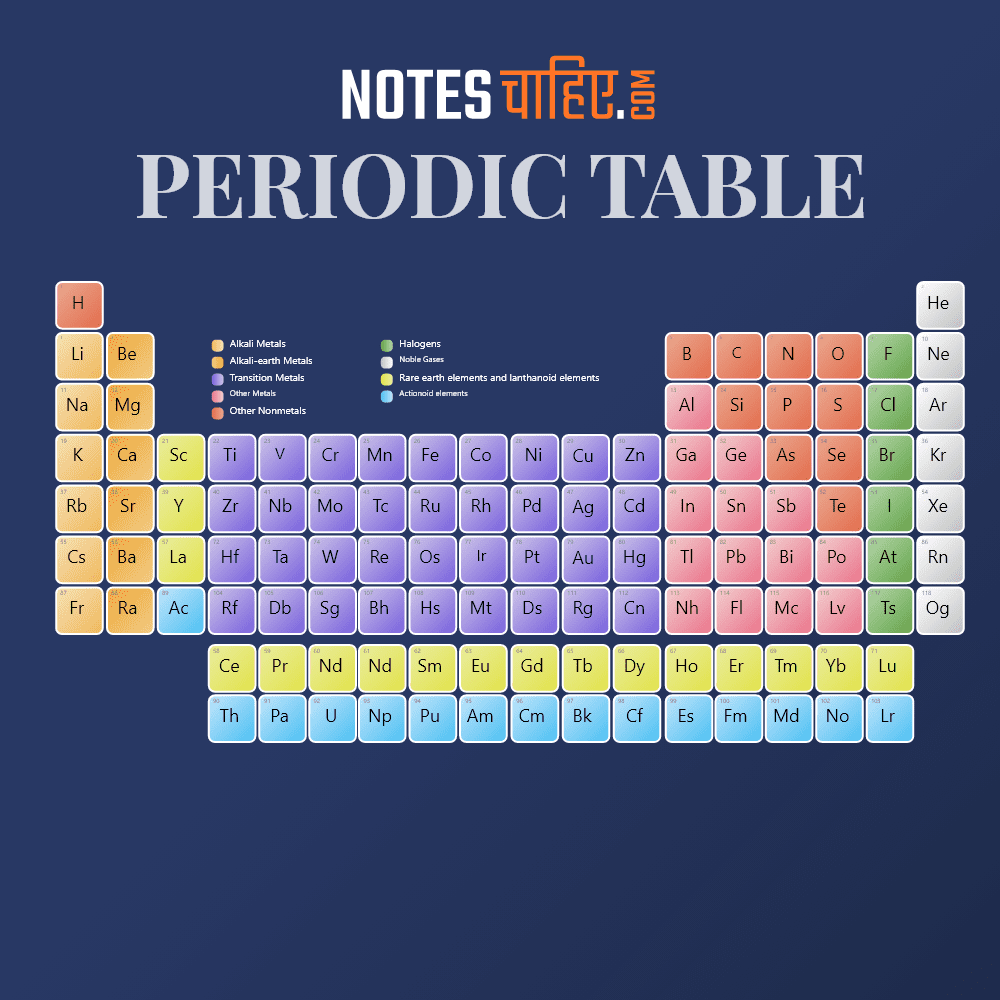
Periods and Groups
The modern periodic table class 10 consists of periods (horizontal rows) and groups (vertical columns). There are a total of seven periods and numerous groups.
Elements within the same group share similar chemical properties, while elements in the same period have the same number of electron shells.
Atomic Number and Atomic Mass
The number of protons in an atom’s nucleus, or atomic number, determines the order of the elements in the periodic table.
Each element takes into protons and neutrons and also shows its atomic mass. Knowledge of atomic numbers and mass aids in identifying and comparing elements.
Atomic Number and Atomic Mass
| Element | Atomic Number | Atomic Mass |
|---|---|---|
| Hydrogen | 1 | 1.008 |
| Helium | 2 | 4.0026 |
| Lithium | 3 | 6.94 |
| Beryllium | 4 | 9.0122 |
| Boron | 5 | 10.81 |
| Carbon | 6 | 12.01 |
| Nitrogen | 7 | 14.01 |
| Oxygen | 8 | 16.00 |
| Fluorine | 9 | 19.00 |
| Neon | 10 | 20.18 |
Valence Electrons
Valence electrons are the electrons present in the outermost energy level of an atom.
The number of valence electrons influences an element’s reactivity and chemical bonding behaviour.
Elements in the same group have the same number of valence electrons, leading to similar chemical properties.
Metallic and Non-Metallic Properties
Elements in the periodic table can be broadly classified as metals, non-metals, and metalloids. Metals are typically good conductors of heat and electricity, whereas non-metals have poor conductivity.
Metalloids exhibit properties of both metals and non-metals.
The periodic table provides a visual representation of these categories.
Periodic Trends
The periodic table also reveals various trends in element properties as we move across periods and down groups.
Atomic Radius
Atomic radius refers to the size of an atom. Generally, the atomic radius decreases as we move from left to right across a period due to increased nuclear charge.
However, atomic radius increases as we move down a group due to the addition of new electron shells.
Ionization Energy
The energy needed to ionize an atom is equivalent to removing an electron from an atom.
The strength of the attraction between the positively charged nucleus and the negatively charged electrons causes the ionisation energy to normally rise from left to right during a time.
Due to the higher shielding effect as we proceed down a group, ionisation energy drops.
Electronegativity
The ability of an atom to draw electrons into a chemical connection is known as electronegativity.
A period’s electronegativity typically rises from left to right as atoms exert a larger attraction on electrons.
As we proceed down a group, electronegativity falls as a result of the growing separation between the nucleus and valence electrons.
Conclusion
The periodic classification of elements is a crucial tool in chemistry that helps us understand the properties and behavior of different elements.
From the early attempts at classification by John Newlands to the groundbreaking work of Dmitri Mendeleev, the periodic table has evolved to provide a comprehensive framework for organizing elements based on their atomic properties.
By studying the modern periodic table and its trends, scientists can make predictions, discover new elements, and deepen our understanding of the building blocks of matter.s
Class 10 Science Notes
Chapter of class 10 science basically includes some important Chapter topics from the NCERT Book, Such as acids bases and salts, metals and non-metals, chemical reactions and equations, Carbon and its Compounds, Life Processes, Control, and Coordination, etc.
Chapter-wise notes on these subjects are available in the table below, click on the links to access them.
- Chapter 1 Chemical Reactions and Equations Class 10 Notes
- Chapter 2 Acids Bases and Salts Class 10 Notes
- Chapter 3 Metals and Non-metals Class 10 Notes
- Chapter 4 Carbon and its Compounds Class 10 Notes
- Chapter 5 Periodic Classification of Elements Class 10 Notes
- Chapter 6 Control and Coordination Class 10 Notes
- Chapter 7 How do Organisms Reproduce Class 10 Notes
- Chapter 8 Heredity and Evolution Class 10 Notes
- Chapter 9 Light Reflection and Refraction Class 10 Notes
- Chapter 10 Human Eye and Colourful World Class 10 Notes
- Chapter 11 Electricity Class 10 Notes
- Chapter 12 Magnetic Effects of Electric Current 10 Notes
- Chapter 13 Our Environment Class 10 Notes
FAQ’s Periodic Classification of Elements
There are seven periods in the periodic table.
Valence electrons determine an element’s reactivity and its ability to form chemical bonds.
Fluorine has the highest electronegativity among all the elements.
Atomic radius increases as we move down a group due to the addition of new electron shells.

