Class 10 Science Chapter 1 Chemical Reactions & Equations Activity 1.1 is very important for both experiment and exam purpose. Here we have explained the activity making NCERT easy.
You can get full explanation about this experiment: Purpose, Materials, Methods, Observation and the conclusion.
Read complete activity so that you can understand better. You can download the full PDF for free at the end of the content.
Class 10 Activity 1.1 Burning Of A Magnesium Ribbon In Air
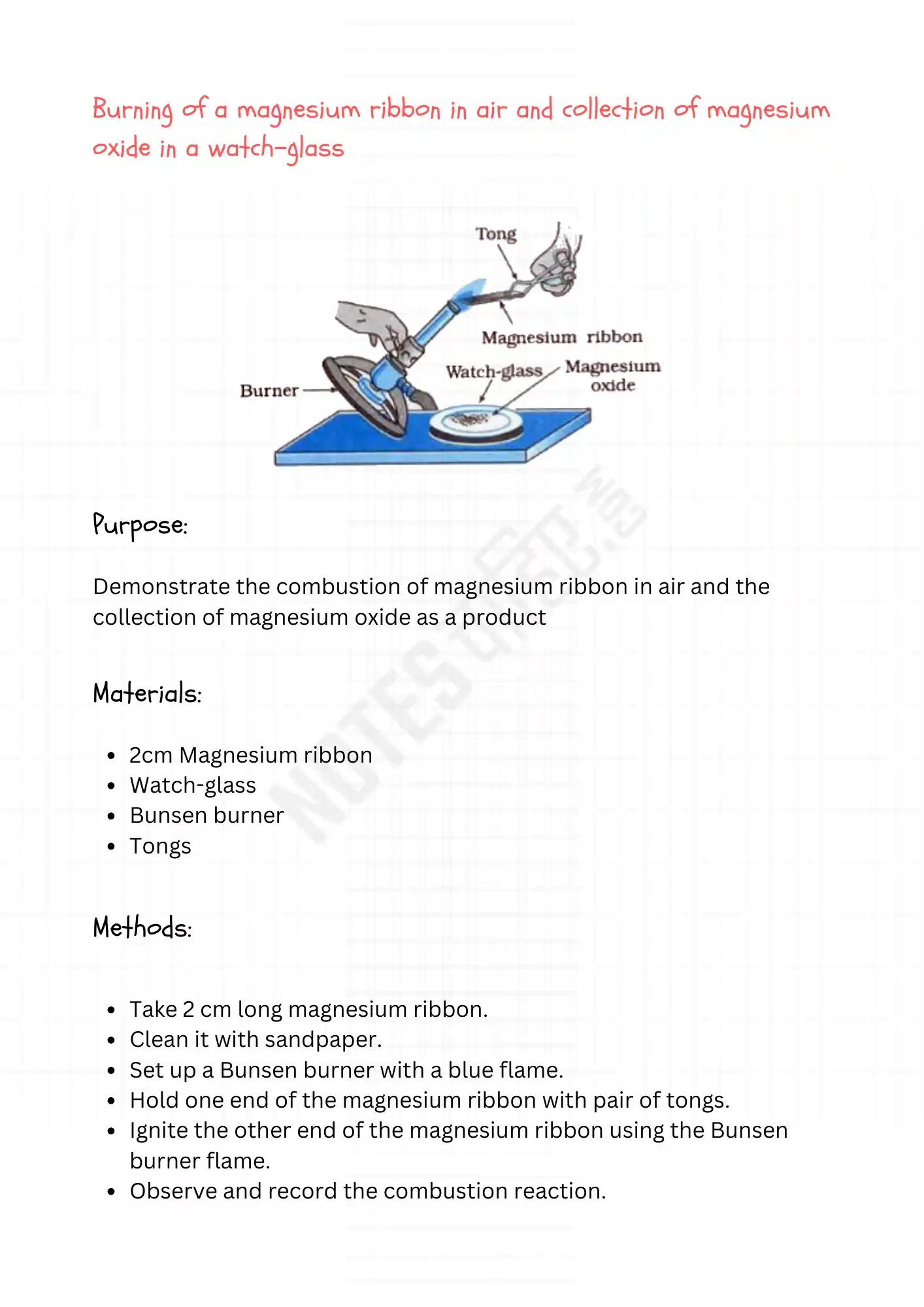
Burning of a magnesium ribbon in air and collection of magnesium oxide in a watch-glass

Purpose:
Demonstrate the combustion of magnesium ribbon in air and the collection of magnesium oxide as a product.
Materials:
- 2cm Magnesium ribbon
- Watch-glass
- Bunsen burner
- Tongs
Methods:
- Take 2 cm long magnesium ribbon.
- Clean it with sandpaper.
- Set up a Bunsen burner with a blue flame.
- Hold one end of the magnesium ribbon with pair of tongs.
- Ignite the other end of the magnesium ribbon using the Bunsen burner flame.
- Observe and record the combustion reaction.
Observation:
- Magnesium ribbon burns with a bright white flame.
- Formation of a white powdery substance in the watch-glass.
- Intense heat released during combustion.
- No residue left on the watch-glass after the reaction.
Results:
- Combustion of magnesium ribbon produces magnesium oxide.
- Magnesium oxide appears as a white powder in the watch-glass.
Conclusion:
The experiment successfully demonstrates the combustion of magnesium in air.
- The formation of magnesium oxide confirms the occurrence of the chemical reaction
2Mg + O2 → 2MgO
- The bright white flame observed indicates the exothermic nature of the reaction, releasing intense heat.
- Absence of residue on the watch-glass suggests complete combustion.
FAQs On Activity 1.1 Burning Of Magnesium Ribbon
The magnesium ribbon which we use usually has a coating of ‘magnesium oxide’ on its surface which is formed by the slow action of oxygen of air on it. So, before burning in air, the magnesium ribbon is cleaned by rubbing with a sand paper. This is done to remove the protective layer of magnesium oxide from the surface of magnesium ribbon so that it may readily combine with the oxygen of air (on heating).
Magnesium is a very reactive metal,so when usually exposed to air it reacts with oxygen and a layer of magnesium oxide is deposited on it. Magnesium oxide is quite stable, so prevents further reaction with oxygen.
2Mg + O2 → 2MgO
The dazzling (very bright) white light given out during the burning of magnesium ribbon is harmful to the eyes. So, the magnesium ribbon should be burned by keeping it as far as possible from the eyes.

