This article provides an in-depth analysis of Carbon and its compounds in Class 10 NCERT solutions. NCERT solutions are designed by the most qualified teachers in India to assist students in Class 10 to achieve high marks in the subject. NCERT solutions for Class 10 science provide a solid basis for each concept. By working on NCERT solutions, students can ensure a smooth and comprehensive comprehension of the advanced concepts.
The CBSE marking scheme has determined that NCERT solutions for Chapter 4 Carbon and its Compounds in Class 10 Science will be of great importance in competitive examinations such as JEE and NEET.
NCERT Solutions for Class 10 Sciences Chapter 4 Carbon And Its Compounds
Before going into the details of the NCERT Solutions for Class 10 Science Chapter 4 Carbon and its compounds, let us first have a list of the units and sub-units under NCERT Solutions for Class 10 Science Carbon and its Compounds.
- Carbon and its compounds
- Bonding in carbon – the covalent bond
- Chemical properties of carbon compounds
- Some important carbon compounds – ethanol and ethanoic acid
- Soaps and Detergents
- Carbon and its Compounds Notes Class 10
- Maths Important Questions for Class 10 NCERT (PDF)
- Notes Kaise Banaye: Top 5 स्टडी नोट्स बनाने के सही तरीके
Class 10 Sciences Chapter 4 Carbon And Its Compounds
Free download NCERT solutions for class 10 science Chapter 4 Carbon and its compounds PDF in Hindi and English Medium. NCERT books based on the updated CBSE syllabus for session 2022-23.
NCERT Solutions for Class 10 Science Chapter 4 Textbooks Question Answer
Question 1
Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds
Answer:
(b) 7 covalent bonds.
Question 2
Butanone is a four-carbon compound with the functional group
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
Answer:
(c) Ketone.
Question 3
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Answer:
(b) The fuel is not burning completely.
Question 4
Explain the nature of the covalent bond using the bond formation in CH3Cl.
Answer:
A covalent bond is formed by the sharing of electrons so that the combining atoms complete their outermost shell.
In CH3Cl: C = 6, H = 1 and Cl = 17 And their electronic configuration is C – 2,4, H – 1 and Cl – 2, 8, 7

Three hydrogen atoms complete their shells by sharing three electrons (one electron each) of carbon atoms.
Chlorine completes its outer shell by sharing one out of seven electrons with one electron of a carbon atom.
Thus carbon atom shares all its four electrons with three hydrogen atoms and one chlorine atom and completes its outermost shell and single covalent bonds are formed in CH3Cl.
Question 5
Draw the electron dot structures for
(a) ethanoic acid
(b) propanone
(c) H2S
(d) F2.
Answer:
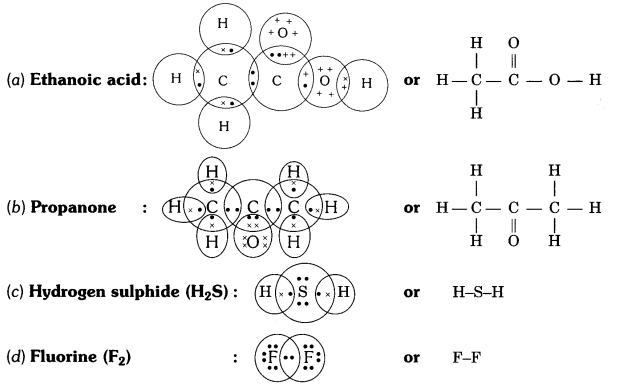
Question 6
What is a homologous series? Explain with an example.
Answer:
Homologous series: A homologous series is a group of organic compounds having
similar structures and similar chemical properties in which the successive compounds differ by -CH2 group.
Characteristics of homologous series :
(i) All members of a homologous series can be represented by the same general formula. For example, the general formula of the homologous series of alkanes is CnH2n+2, in which ‘n’ denotes the number of carbon and hydrogen atoms in one molecule of alkane.
(ii) Any two adjacent homologues differ by one carbon atom and two hydrogen atoms in their molecular formulae.
(iii) The difference in the molecular masses of any two adjacent homologues is 14u.
(iv) All the compounds of a homologous series show similar chemical properties.
(v) The members of a homologous series show a gradual change in their physical properties with an increase in molecular mass.
For example, the general formula of the homologous series of alkanes is CnH2n+2, in which ‘n’ denotes the number of carbon atoms in one molecule of alkane. Following are the first five members of the homologous series of alkanes (general formula CnH2n+2).
| Value of n | Molecular formula | Name of compound |
| 1 | CH4 | Methane |
| 2 | C2H6 | Ethane |
| 3 | C3H8 | Propane |
| 4 | C4H10 | Butane |
| 5 | C5H12 | Pentane |
Question 7
How can ethanol and ethanoic acid he differentiated on the basis of their physical and chemical properties?
Answer:
Difference on the basis of physical properties
| Property | Ethanol | Ethanoic acid |
| (i) State | Liquid | Liquid |
| (ii) Odour | Sweet smell | Pungent vinegar-like smell |
| (iii) Melting point | 156 K | 290 K |
| (iv) Boiling point | 351 K | 391 K |
Difference on the basis of chemical properties
| Test | Ethanol | Ethanoic acid |
| (i) Litmus test | Does not happen. | Blue litmus solution turns red. |
| (ii) Sodium hydrogen carbonate test | C2H5OH + NaHCO3 → No reaction No brisk effervescence. | CH3COOH + NaHCO3 → CH3COONa + H2O + CO2 Brisk effervescence due to evolution of CO2. |
| (iii) Alkaline potassium permanganate | No change in the colour of the litmus solution. | Does not happen so. |
Question 8
Why does micelle formation take place when soap is added to water? Will a micell be formed in other solvents such as ethanol?
Answer:
When you add soap to water, something interesting happens. It’s all about the soap molecules. You see, the soap molecules have two different parts. One part is like a water-hater, it doesn’t mix well with water (we call it hydrophobic). The other part, on the other hand, is a water-lover, so it dissolves easily in water (we call it hydrophilic). So, when you put soap in water, these molecules come together to form something called a micelle. It’s like a little club where the hydrophobic parts stick together on the inside, while the hydrophilic parts face the water. This is what makes soap work its magic when you’re cleaning things
Question 9
Why are carbon and its compounds used as fuels for most applications?
Answer:
Carbon and its compounds give a large amount of heat per unit weight and are, therefore, used as fuels for most applications.
Question 10
Explain the formation of scum when hard water is treated with soap.
Answer:
Calcium and magnesium salts are present in hard water. When calcium and magnesium react with soap, they produce an insoluble precipitate. The formation of scum diminishes the cleansing properties of soap in hard water.
Question 11
What change will you observe if you test soap with litmus paper (red and blue)?
Answer:
Red litmus Paper will turn blue because soap is alkaline in nature and Blue litmus Paper remains blue in the soap solution.
Question 12
What is hydrogenation? What is its industrial application?
Answer:
Hydrogenation is the process of adding hydrogen to unsaturated hydrocarbons to form a saturated hydrocarbon. Hydrogenation is carried out using nickel (Ni)–based catalysts and palladium (PD) based catalysts.

Application: The process of hydrogenation has an important industrial application. It is used to prepare vegetable ghee (or vanaspati ghee) from vegetable oils.
Question 13
Which of the following hydrocarbons undergo addition reactions: C2H6, C3H8, C3H6, C2H2 and CH4
Answer:
Addition reactions take place only in unsaturated hydrocarbons. So, the addition reaction takes place only in C3H6 and C2H2.
Question 14
Give a test that can be used to differentiate chemically between butter and cooking oil.
Answer:
Unsaturated compounds decolor bromine and saturated compounds do not. So, chemically, we can tell a cooking oil from butter by the presence of bromine in the water. So, add a small amount of bromine to cooking oil and add butter in separate test tubes.
- Cooking oil decolourises bromine water showing that it is an unsaturated compound.
- Butter does not decolourise bromine water showing that it is a saturated compound.
Question 15
Explain the mechanism of the cleaning action of soaps. OR Explain the cleansing action of soaps.
Answer:
When you put a dirty cloth in water with dissolved soap, the soap molecules attached to the hydrocarbon ends of the micelle will attach to the oily or grease particles on the dirty cloth surface. The soap micelle uses its hydrocarbon end to entrap the oily/greasy particles. However, the ionic end of soap molecules attached to micelles will remain attached to the water.

Class 10 Carbon And Its Compounds MCQ for Board Exam
- Which element is the basis for organic chemistry?
A. Hydrogen
B. Carbon
C. Oxygen
D. Nitrogen
Answer: B. Carbon - How many valence electrons does carbon have?
A. 2
B. 4
C. 6
D. 8
Answer: B. 4 - What is the molecular formula of methane?
A. CH2
B. CH3
C. C2H4
D. CH4
Answer: D. CH4 - Which type of bonding is primarily found in organic compounds?
A. Ionic
B. Covalent
C. Metallic
D. Hydrogen
Answer: B. Covalent - What is the term for compounds containing only carbon and hydrogen atoms?
A. Organic compounds
B. Inorganic compounds
C. Carbonates
D. Hydrocarbons
Answer: D. Hydrocarbons - What is the simplest alkane?
A. Methane
B. Ethane
C. Propane
D. Butane
Answer: A. Methane - The process of converting alkenes to alkanes is called:
A. Hydrogenation
B. Halogenation
C. Oxidation
D. Polymerization
Answer: A. Hydrogenation - Which functional group is present in alcohols?
A. Carbonyl
B. Hydroxyl
C. Carboxyl
D. Ester
Answer: B. Hydroxyl - What is the general formula for alkanes?
A. CnH2n
B. CnH2n+2
C. CnHn
D. CnHn-2
Answer: B. CnH2n+2 - Which gas is released during the combustion of hydrocarbons?
A. Oxygen
B. Nitrogen
C. Carbon dioxide
D. Hydrogen
Answer: C. Carbon dioxide - What is the main component of natural gas?
A. Methane
B. Ethane
C. Propane
D. Butane Answer:
Answer: A. Methane - Which type of isomerism is exhibited by compounds with the same molecular formula but different connectivity of atoms?
A. Structural isomerism
B. Geometric isomerism
C. Optical isomerism
D. Tautomeric isomerism
Answer: A. Structural isomerism - What is the functional group in a carboxylic acid?
A. Hydroxyl
B. Carbonyl
C. Carboxyl
D. Alkyl
Answer: C. Carboxyl - Which organic compound is known as the “building block of life”?
A. Proteins
B. Carbohydrates
C. Nucleic acids
D. Carbon
Answer: D. Carbon - What is the term for a molecule with the same molecular formula but different spatial arrangement of atoms?
A. Stereoisomer
B. Homologue
C. Isobar
D. Allomer
Answer: A. Stereoisomer - What type of bonding is found in diamond?
A. Covalent
B. Ionic
C. Metallic
D. Hydrogen
Answer: A. Covalent - Which element is often used as a dopant to produce p-type semiconductors in carbon materials?
A. Nitrogen
B. Silicon
C. Boron
D. Phosphorus
Answer: C. Boron - What is the process of converting crude oil into useful hydrocarbons called?
A. Fractional distillation
B. Cracking
C. Polymerization
D. Condensation
Answer: B. Cracking - Which hydrocarbon has a triple bond between carbon atoms?
A. Alkane
B. Alkene
C. Alkyne
D. Arenes
Answer: C. Alkyne - What is the functional group in aldehydes?
A. Hydroxyl
B. Carbonyl
C. Carboxyl
D. Alkyl
Answer: B. Carbonyl - What is the main carbon compound found in the cell walls of plants?
A. Cellulose
B. Starch
C. Chitin
D. Glycogen
Answer: A. Cellulose - Which type of carbon is used in water purification filters due to its high surface area?
A. Graphene
B. Fullerene
C. Diamond
D. Charcoal
Answer: D. Charcoal - Which of the following is not a common use of carbon compounds?
A. Fuel
B. Medicine
C. Building materials
D. Clothing
Answer: C. Building materials - In the carbon cycle, what is the primary process by which carbon is returned to the atmosphere?
A. Photosynthesis
B. Respiration
C. Decomposition
D. Combustion
Answer: D. Combustion - What is the process of converting a liquid to a gas at the boiling point called?
A. Condensation
B. Sublimation
C. Evaporation
D. Melting
Answer: C. Evaporation - Which type of carbon compound is responsible for the structure and rigidity of exoskeletons in insects and the shells of crustaceans?
A. Proteins
B. Carbohydrates
C. Chitin
D. Lipids
Answer: C. Chitin - What is the term for the process in which green plants use sunlight to convert carbon dioxide and water into glucose and oxygen?
A. Photosynthesis
B. Respiration
C. Fermentation
D. Combustion
Answer: A. Photosynthesis - Which type of hydrocarbon has alternating double bonds in the carbon chain?
A. Alkane
B. Alkene
C. Alkyne
D. Aromatic hydrocarbon
Answer: D. Aromatic hydrocarbon - What is the term for the study of carbon compounds?
A. Geology
B. Biology
C. Chemistry
D. Carbonology
Answer: C. Chemistry - What is the term for a carbon compound with the same molecular formula but different structural arrangement of atoms?
A. Isomer
B. Homolog
C. Isotope
D. Polymorph
Answer: A. Isomer
