NCERT Solutions for Class 10 Science all chapters in Hindi and English Medium updated for new session 2023-24. According to the revised syllabus and rationalised NCERT books issued for the academic year 2023-24, there are only 13 chapters in 10th science. If you are planning to Score high in Class 10 Science-based NCERT solutions, follow the two months of preparation for 10th science. Class 10th Science solutions solve problems related to the science curriculum provided by the National Council of Educational Research and Training (NCERT) in Bharat.
NCERT Solutions for Class 10 Science
Class 10 Science | Chemistry
Chapter 1: Chemical Reactions and Equations
Chapter 2: Acids, Bases and Salts
Chapter 3: Metals and Non-Metals
Chapter 4: Carbon and its Compounds
Class 10 Science | Biology
Chapter 5: Life Processes
Chapter 6: Control and Coordination
Chapter 7: How do Organisms Reproduce?
Chapter 8: Heredity
Class 10 Science | Physics
Chapter 9: Light – Reflection and Refraction
Chapter 10: Human Eye and Colourful World
Chapter 11: Electricity
Chapter 12: Magnetic Effects of Electric Current
Class 10 Science | Environmental Studies
Chapter 13: Our Environment
Class 10 Science NCERT Solutions Include Various Scientific Concepts and Topics That Students Study In Their 10th Grade Science Book. Class 10 NCERT Solutions are useful for Students and Educators. These solutions provide structured guidance for teaching scientific concepts, problem-solving and exam preparation. These solutions are available in NCERT official textbooks, educational websites and online platforms.
Other NCERT Resources for Class 10 Science
In addition to the study materials provided by NCERT for Class 10, students can also avail of other study materials such as NCERT class 10 science example, NCERT science textbook, NCERT science syllabus, NCERT science solutions, etc. All of these study materials are prepared according to the CBSE science syllabus for Class 10 and can be used by students to prepare for the board exam. Students can avail these study materials once they have completed the complete syllabus which will help them to revise the syllabus quickly before the examination.
Here are the links to other NCERT resources for Class 10 science that can help you prepare well for the Class 10 board exam.
NCERT Class 10 Science Notes
NCERT Textbook Class 10 Science
Best Notes Making Format, Ideas And Examples 2024
Rationalised NCERT Book Solutions Class 10 Science All Chapters Brief:
Chapter 1 – Chemical Reactions and Equations
In the first chapter, the students will learn about chemical reactions, equations, conducting combination and decay reactions etc. In the preceding chapters, the students have learned about the physical and chemical alteration of matter. Whenever there is a chemical change in matter, a chemical reaction is called a complete chemical reaction. Students will learn how to write chemical reactions symbolically. They will also learn how to balance various chemical equations symbolically.
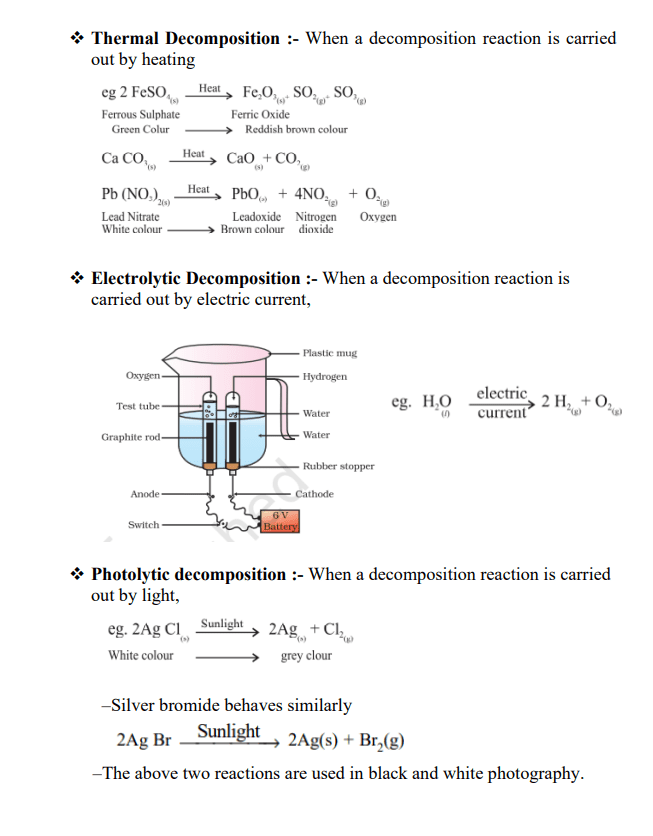
In the next subtopic, students will learn about various chemical reactions like Combination reactions, decomposition reactions and Displacement reactions, as well as various examples and various chemical reactions based on energy. Students will learn about exothermic reactions where heat is passed along with products, while endothermic reaction involves the absorption of energy. Then, they will learn about redox reactions by combining reduction reactions with oxidation reactions. Students will be able to write chemical reactions symbolically with their corresponding chemical equations.
Chapter 2 – Acids, Bases and Salts
In previous classes, students were taught that acid and base are responsible for the sour and bitter taste of food. Acid is acidic and changes the colour of the blue litmus from red to red, while base is bitter and changes the colour from red to blue.
In this chapter, students will learn about acids and bases reactions, acids and bases cancelling each other’s effects, and many other interesting things that are used in everyday life. Students will learn about the chemical properties of acid and base, how acids and base react with metals, how metal carbonate and metal hydrogen carbonate react with acids, acids and base reactions with each other, and reactions of metallic oxides and non-metallic oxides with acids explained with appropriate examples and different chemical reactions. Students will also learn about the commonalities of all acids and bases with a suitable example.
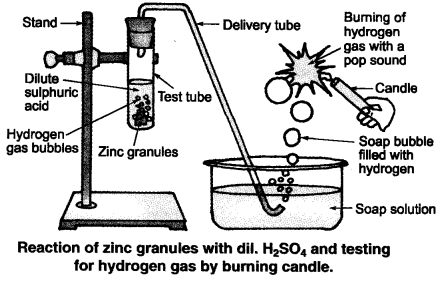
The conclusion of this chapter is that acid solution conducts electricity in water. In this chapter, students will be able to perform various experiments to understand what happens when acid or a base is added to a water solution, and how strong acid or base solutions are using universal indication. Students will also be able to understand the significance of pH in their daily life. The chapter concludes with an in-depth description of salt preparation, its properties and uses.
Chapter 3 – Metals and Non-metals
In previous classes, students have had to learn about different elements that are classified as metal or non-metal based on their properties. In Class 10 Science Chapter 3, students will be learning about the physical characteristics of metal and non-metal.
Metals are shiny, elastic, ductile, conductive and solid at room temperature. Apart from mercury which is liquid, all other metals are solid. The physical characteristics of metals are discussed on various parameters like ductility, elasticity, tensile, strength etc.
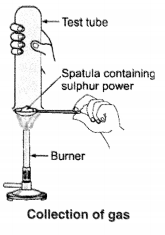
Non-metals, on the other hand, are different from metals and are classified according to their properties. For example, some examples of Non-Metals are Carbon, Sulfur, Iodine, Oxygen, Hydrogen, etc.
Under the subtopic Chemical Properties of Metals, students will have to learn about chemical reactions with oxygen gas water, acid, etc. Reactions and conditions are determined by the reaction series. Potassium is the most reactive reaction series and gold is the least reactive reaction series. Electrovalent compounds are formed when electrons are transferred from metal to non-metal.
General properties of ionic compounds
Physical nature
Melling and boiling points
Solubility
Conduction of electricity
Metallurgy
Metallurgy is the process of extracting metals from their ore and refining them for their use
Metals are refined using the electrolytic refining process
Corrosion
The final topic
Chapter 4 – Carbon and its Compounds
In the last chapter, we looked at compounds that are important to us. This chapter will look at some of the more interesting compounds and some of their properties. We will also look at carbon, an element that is very important to us both as an element in its elemental form and as a combined element. Carbon is one of the most versatile elements that makes up the basis of all living things and a lot of the things that we use.
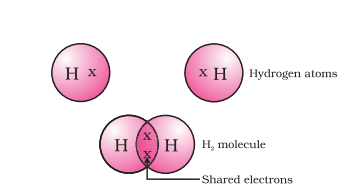
Covalent bonds are created when two atoms share electrons so that they can form a covalent bond with each other and with other elements like hydrogen, oxygen, sulfur, nitrogen, chlorine, etc.
Organic compounds can be divided into saturated, unsaturated carbon compounds and compounds with a single bond. Saturated carbon compounds have a double or a triple bond. Examples of saturated carbon and hydrogen compounds include:
Methane
Ethane
Propane
Butane
Pentane
Hexane
Ethanoic Acid
Chapter 5 – Life Processes
Life Processes are the six life processes performed by all living organisms. NCERT Class 10 Science Chapter 5 explains these six life processes as follows:
Movement
Respiration
Growth
Reproduction
Egestion
Nutrition is the process of eating, digesting, absorbing, transporting, assimilating and excretion of food.
Nutrition is further classified as Autotrophic Nutritional and heterotrophic Nutritional.
Autotrophic Nutritional is the process of consuming simple inorganic material from the environment.
Heterotrophic Nutrients are the processes of consuming complex high energy organic material from other organisms.
Parasitic Nutrients are the process of eating other organisms’ nutrients.
Nutritional in Human Beings
The different stages of nutrition are:
Ingestion
Digestion
Oesophagus
Stomach
Small intestine
Bile
Apothecia
Respiratory
Chapter 6 – Control and Coordination
Class 10, Chapter 6 deals with control and coordination. Control and coordination refer to the functions of our nervous system and the hormones in our body. The reactions of our nervous system are called reflex actions, voluntary actions or involuntary actions.
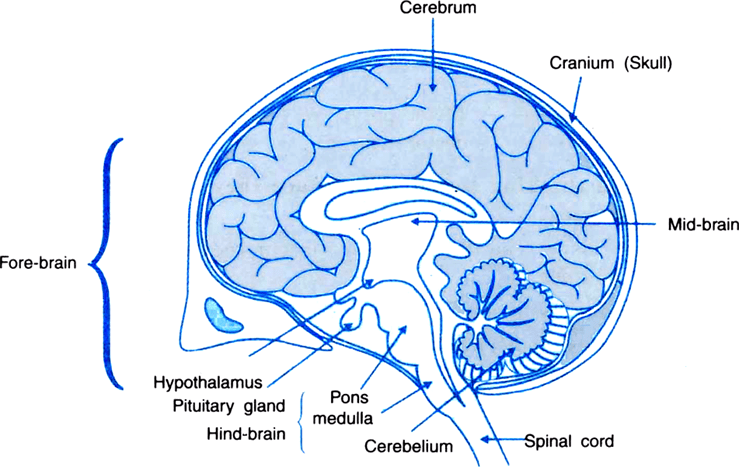
The nervous system transmits information through electrical impulses. It receives information from the sense organs. It acts through the muscles.
Chemical coordination is present in both plant and animal organisms. The hormones produced by one part of the body move to the other part to produce the desired action. The feedback mechanism controls the actions of the hormones.
Chapter 7 – How do Organisms Reproduce
Unlike other forms of life, reproduction is not necessary to sustain an organism’s life. How do organisms reproduce Chapter involves a DNA copy and extra cellular apparatus produced by the cell that carries out the reproductive process. Depending on the structure of their body, different organisms use different reproductive mechanisms. For example, in fission, many bacterial and protozoan cells simply divide into a single or multiple daughter cells. Hydra, for example, can regenerate if it is broken into pieces. It can also produce buds that grow into new individuals.

In some plants, roots, stems and leaves develop into new plants by vegetative propagation. This is an example of asexual reproduction, where new generations are generated from a single organism. Sexual reproduction involves having two individuals for the purpose of creating a new individual. The DNA copying mechanisms produce variations that are useful for the survival of a species. The modes of sexual reproduction allow more variation to be generated.
Chapter 8 – Heredity And Evolution
In this chapter, we’ll look at Heredity and evolution. We’ve looked at how reproductive processes create new individuals that are alike, but slightly different. We’ve looked at how some variation is produced during asexual reproduction, and we’ve talked about the rules for the inheritance of traits in human beings. The rules for inheritance of traits relate to both father and mother contributing about the same amount of genetic material to a child. That means each trait can be affected by both father and mother DNA. Sex is determined by various factors in different species. Changes in non-reproductive tissue caused by environmental factors aren’t inherited. Speciation occurs when variation is combined with geographic isolation. Evolutionary relations are traced in the taxonomy of organisms. tracing common ancestors back in history leads us to the conclusion that at some time non-living matter must have created life.
Chapter 9 – Light Reflection and Refraction
In Class 10 Science of NCERT, we will explore the phenomenon of Reflection and Refraction of Light using Straight-line Propagation of Light. These Basic Concepts will help us to study some of the Optical Phenomena in nature. In this chapter, we will discuss the Reflection of Light by Spherical Mirrors, Refraction of Light and their Application in Real Life. Light is the source of energy that gives us the sensation of seeing in our eyes. The light seems to travel straight lines. We will learn about the different types of Spherical Mirrors like Convex and Concave. Various terms related to Spherical Mirrors such as Centre of Curriculation, ROC, etc., Focus, Pole, etc., Ray Diagrams, Uses of Spheres Mirrors, Mirror Formula, Relationship between Object-Distance, Image-Distance, and Focal Length of Spheric Mirrors. The Focal length of a Spheric Mirror is equal to Half of Its ROC.
Chapter 10 – The Human Eye and Colorful World
In the previous chapter we learned about light and some properties of light. In this chapter, we will look at some of the optical phenomena in nature. We will also look at the rainbow formation, the splitting of white light, and the blue colour in the sky.
Our eyes are one of the most important and sensitive sense organs we have. They allow us to see the beautiful world around us and the colours we see. The ability of our eyes to focus on near and distant objects by adjusting their focal length is known as the accommodation of our eyes.
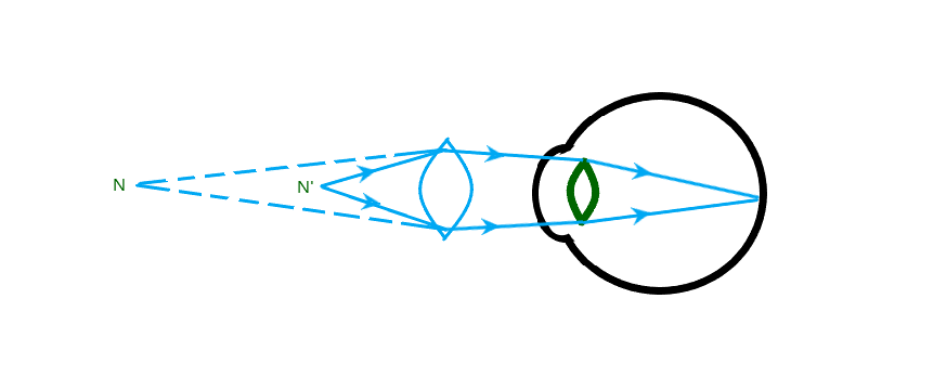
The smallest distance at which our eyes can see objects without strain is known as the near point of our eye. This is the smallest distance at which we can see something clearly without strain. For an adult with normal vision this would be around 25cm.
Common refractive defects of our eyes include myopia, hypermetropia, presbyopia, and short-sightedness.
Myopia is the inability to focus on distant objects. In this case, the image of the distant object is focused before it is transmitted to the retina before it is corrected by a concave lens with suitable power.
Chapter 11 – Electricity
Electricity plays a vital role in contemporary society. It’s a versatile and easy-to-use form of energy that can be used for a wide range of purposes in homes, educational institutions, hospitals, industrial plants, etc.
Electricity is a phenomenon of charge flow. An electric current is a stream of electrons flowing through a conductor. The direction of the current is usually opposite to that of the direction of the flow of the electrons.
The SI unit of electrical current is the ampere (ampere).
To set up the electrons in an electrical circuit, we need a cell or battery. The potential difference across a cell’s terminals is called a potential difference. This potential difference is measured as a volt (V).
Resistance is a property of a conductor that prevents the current from flowing through it. Resistance determines the size of the current, and the SI unit for resistance is the ohm (ohm).
The resistance of a resistor depends directly on the length of the conductor, and inversely on the cross-section of the conductor. The temperature of the resistor is proportional to the resistance of the current through the resistor.
Chapter 12 – Magnetic Effects of Electric Current
Students will learn about magnetic fields and electromagnetic effects in this chapter, as well as electromagnets, electric motors, and electric generators.
What is a compass needle?
A compass needle is a tiny magnet with one end pointing north and the other side pointing south. The north pole is called the north pole and the south pole is called the south pole. There is a magnetic field in the area around the magnet. The force of the magnet is detected.
How do you represent a magnetic field?
A field line is the path along which a free north pole would
The magnetic field that occurs around a conductor when an electric current flows through it is determined by its shape. A current-carrying solenoid has a magnetic field that is similar to the one that a bar magnet has.
An electromagnet is made up of a hard iron core wrapped around an insulated copper wire coil. A current-carrying conductor experiences a force when placed under a magnetic field.
Chapter 13 – Our Environment
In this chapter, we look at how different parts of the environment work together and how we interact with them. The different parts of an ecosystem depend on each other. Producers release the energy from the sun into the ecosystem. As we move from one level of food to another, we lose energy. This reduces the number of levels of food in the food chain. We explain the food-chain in detail using examples from nature, e.g. in forests, grasslands and ponds. Human activities affect the environment. For example, the use of chemicals such as chlor-ofluorocarbons (CFCs) has weakened the ozone layer, which protects the ozone layer from the Sun’s ultraviolet radiation. The waste that we produce may be biocompatible, non-biocompatible, or both. Disposing of this waste can cause serious environmental problems.
